The synergistic collaboration between Prof. Zhenyu Zhang’s theory group and Prof. Changgan Zeng’s experiment group has recently made an important progress in synthesis of graphene. They found that enhanced London dispersion forces can drastically reduce growth temperature of graphene on metal substrates, from the typical temperature of ~1000˚C to 300˚C, which was published on 31th May 2013 with the title "Drastic reduction in the growth temperature of graphene on copper via enhanced London dispersion force" (Scientific Reports 3, 1925 (2013)). For the past few years, two groups at USTC‒Prof. Zhang's and Prof. Zeng's groups‒have carried out intense researches on graphene-based nanostructures. Prof. Zhang's group has focused on theoretical researches for design of graphene-based nanostructures on solid surfaces. The theoretical works have contributed to better understandings of graphene syntheses on metal substrates. Prof. Zeng's group has tried to develop an improved fabrication method of low-dimensional graphene based nanostructures, and the low-temperature graphene synthesis is one of the most valuable results of the efforts. The research results of the two groups are published in the most prestigeous journals such as Physical Review Letters, ACS Nano, Carbon, etc.
Graphene is a flat monolayer of carbon atoms tightly packed into a two-dimensional honeycomb lattice. It has a lot of appealing potential applications in many fields due to its exotic mechanical, thermal, optical, and electronic properties. In 2004, British scientists, Andre Geim and Konstantin Novoselov et al. first used the “scotch-tape” approach to obtain graphene samples by mechanically exfoliating graphite, which made both of them awarded the 2010 Nobel Prize for Physics. Since then, many efforts have been devoted to developing diverse approaches to the synthesis of graphene. Among them, the methods using nonequilibrium growth on surfaces have shown great promise for large-area synthesis of highquality graphene films.
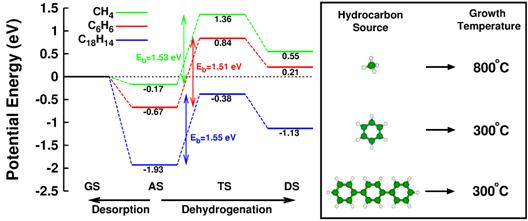
Figure 1: (Left) Energetics and kinetics of competitive adsorption-dehydrogenation reactions for the three different hydrocarbon sources and (right) graphene growth temperatures with respect to the used hydrocarbon sources.
In 2009, the research group leading by Prof. Rodney S. Ruoff at the University of Texas at Austin successfully grew large-area graphene films of the order of centimeters on Cu substrates by chemical vapor deposition (CVD), showing copper is the best catalytic metal substrate for graphene epitaxial growth. Based on first-principles density functional theory calculations, Prof. Zhang and his collaborator, Dr. Wenguang Zhu at the University of Tennessee (he has returned to work full time at USTC), investigated the carbon nucleation behavior in the initial stages of graphene epitaxial growth on stepped metal surfaces and illustrated the copper as a catalytic metal substrate is more efficient for nucleation of carbon islands than other metal substrates (Phys. Rev. Lett. 104, 186101 (2010)). However, such efficient nucleation undesirably introduces grain boundaries (GBs) during graphene growth, which is one standing obstacle facing the community of epitaxial graphene on copper. In order to solve this problem, they designed a superstructured alloyed surface and proposed a two-step kinetic pathway to effectively suppress the formation of GBs (Phys. Rev. Lett. 109, 265507 (2012)). This work opens the door towards a new and viable approach for mass production of single crystalline monolayer graphene. On the other hand, the typical fabrication method requires high temperatures around 1000˚C and the CVD method at such high temperatures is undesirable for many practical reasons. Prof. Zeng's group developed an improved routine of CVD graphene synthesis using liquid benzene as carbon source instead of the commonly used methane gas and found that high quality and monolayer graphene films were achieved at growth temperature as low as 300˚C(ACS Nano 5, 3385–3390 (2011)). It is the lowest-temperature growth of graphene by CVD according to the public report.
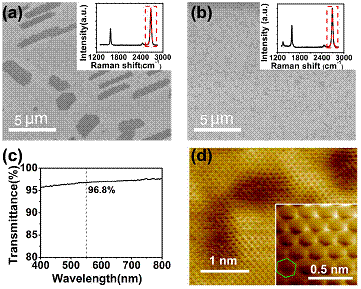
Figure 2: Characterization of the graphene films grown at 300˚C from two different aromatic molecules as carbon sources.
Recently, postdoctoral researcher Dr. Jinho Choi in Prof. Zhang's group and Prof. Zeng collaborate to find the origin of the drastic reduction in growth temperature of graphene on metal substrates (Scientific Reports 3, 1925 (2013)). Dr. Choi predicted theoretically that London dispersion force, which is a major component of van der Waals forces, plays a decisive role in delicate competitive chemical reactions included in the graphene growth. Specifically, enhanced London dispersion force of larger aromatic molecules prevents easy desorption of the adsorbed molecule from the surface, promoting necessary dehydrogenation reactions. This enables the low-temperature graphene growth when we replace the typical carbon source of methane by aromatic molecules with larger sizes (see Figure 1). The theoretical findings are validated by experimental examinations of Prof. Zeng's group. The experiment confirms the low-temperature growth of graphene on Cu substrate at 300˚C using a new aromatic source by the name of p-Terphenyl and extends the scope of application of this mechanism. The experiment also clearly demonstrates that the grown graphene from p-Terphenyl is predominantly monolayer in thickness, by using Raman, optical transmittance, and scanning tunneling microscopy measurements (see Figure 2). Because the London dispersion force is ubiquitous in nature due to universal existence of transient dipole, which is the source of the London dispersion force, the general trend established in this work is applicable to broader areas of nanostructure fabrications on metal substrates.
These works were supported by National Natural Science Foundation of China, Chinese Academy of Sciences Fellowships for Young International Scientists, Oversea Postdoctoral Fellowship of National Research Foundation of Korea, and Ministry of Science and Technology of China.
